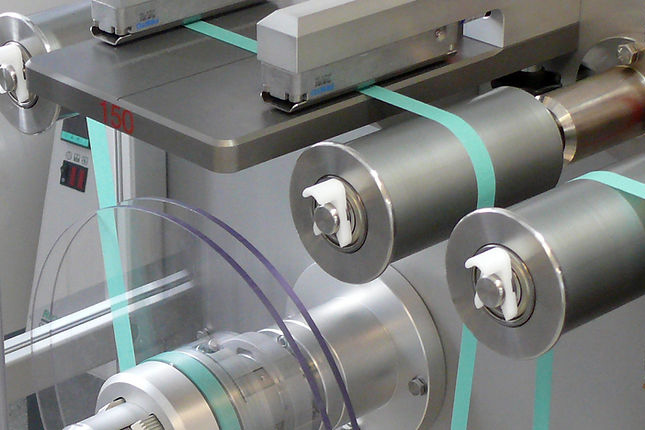
Technologies
Competence center 2D & 3D printing technology
We offer following services in printed drug formulations
- Development of sophisticated formulation concepts using printing techniques
- Conduct of feasibility studies (proof of concept) for new molecular entities
- Filament and ink formulations
- Dose-finding studies using 2D printing
- Lifecycle management
- Effective network with equipment providers, specialized raw material suppliers and universities
All services are also available for formulations containing high-potent drugs & narcotics and under GMP requirements.
Advantages of printed drug formulation
- Individually manufactured
- Individual doses
- Small batch sizes or single drug batch size possible
- Tailored release characteristics
- Fast on-set
- Combination of APIs not compatible with conventional manufacturing techniques
- Combination of APIs that are not marketed
- Improvement of drug product stability
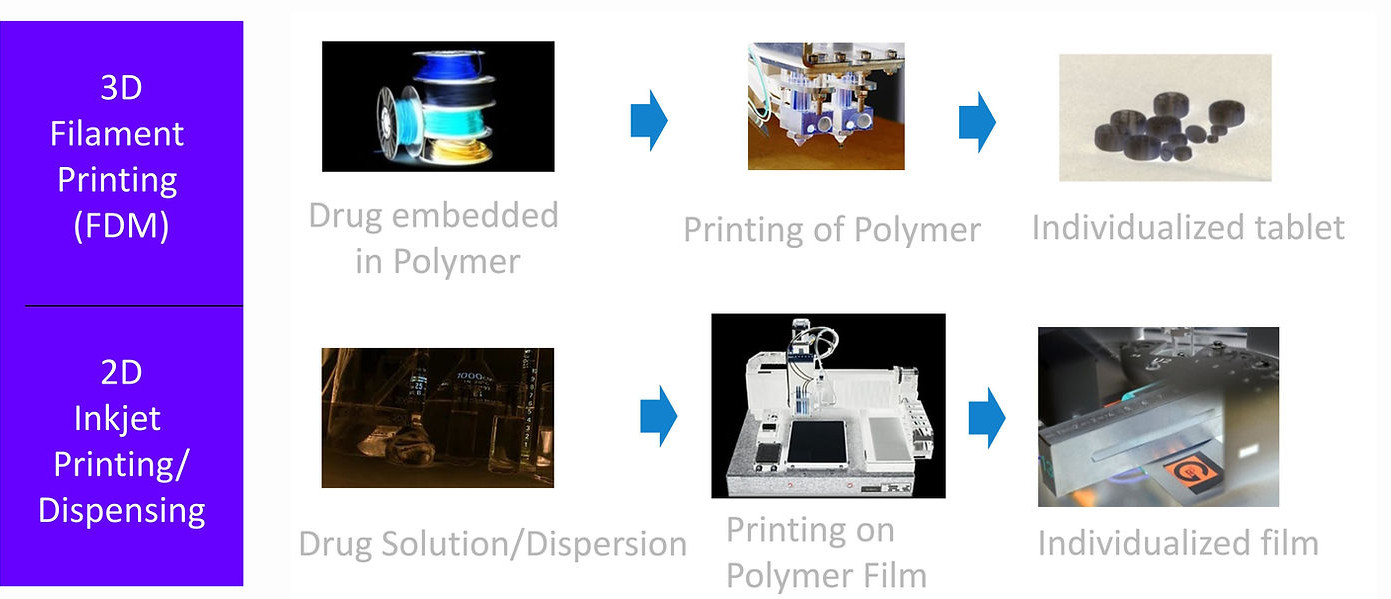
Innovative, cost-effective, individualized dosage forms, small batch sizes –
all possible @ Gen-Plus with the 2D and 3D API printing technology
Personalized medicine
- Tailor-made pharmacotherapies
- With respect to individual health situation
- Considering physiological constitution
- Genetic, metabolic and gender-specific characteristics
Solid Dosage Forms
For the preparation of solid dosage forms we use various technologies. This enables us to meet the particular requirements of the targeted product, e.g. Immediate Release (IR), Modified Release (MR), Controlled Release (CR), Extended Release (ER) / Sustained Release (SR).
A substantial number of products have been successfully developed:
- Tablets (Mono- / Multi-layer Tablets, Effervescent Tablets)
- Film Tablets
- Orodispersible Tablets (sublingual Tablets, super-fast dissolving Tablets)
- Capsules
- Granulates
- Pellets
- Adsorbates
- Lyophilisates
Manufacturing technologies at Gen-Plus include:
- Fluidized bed granulation
- Compaction
- Coating
- Fluidized bed drying
- Hot melt granulation
- Tray drying
- Single pot granulation
- Sieving
- Diffusion blending
- Forced blending
- Grinding
- Spray drying
- Tableting
- Capsule filling
Patches / Oral Thin Films
In the field of transdermal drug delivery systems (TDS) and oral thin films (ODF) we offer development service in the range of small-scale samples up to the manufacturing of phase I clinical samples. We are equipped with a pilot machine for continuous coating, a cutting machine and a punching/packing machine with an output of 25 units / min.
HME – Hot Melt Extrusion
With Hot Melt Extrusion (HME) we offer a continuous production process, which allows improving the bioavailability of challenging APIs (BCS class III and IV).
Micro-/ Nanoemulsions
We offer the development of Micro-/Nanoemulsions and their incorporation in solid dosage forms. These technologies enable the solubilisation of poorly soluble APIs, resulting in an improved bioavailability.
Amorphization of APIs
We offer a wide range of technologies for amorphization like:
- Hot Melt Extrusion
- Spray drying / Embedding
- Freeze-drying
- Precipitation / Co-Precipitation
- Adsorption on the surface of (mesoporous) materials
New Technologies
Gen-Plus is continuously engaged in getting access to innovative pharmaceutical technologies:
PLEASE-Technology is based on controlled on laser perforation of the skin (epidermal stratum corneum), which allows a facilitated transport of topical peptide formulations through the skin.
Using 2D & 3D-Printing-Technology we aim for the development of personalized solid dosage forms regarding dosage and release
profile.
More Information?
Together with our business partners, we develop innovative formulation concepts and new technologies.
+49 89-780179-40
sales@gen-plus.de
Our Solutions
Our technologies at Gen-Plus include:
Questions?
Please use the contact details or form below.
Tel. +49 89-780179-40
Mail: sales@gen-plus.de